Condensate Recovery

Contents
Introduction to Condensate Recovery
An introduction to the reasons for condensate recovery and return, including energy costs, water charges, effluent restrictions and water treatment costs. Includes sample calculations for potential savings
Introduction to Condensate Recovery
Steam is usually generated for one of two reasons:
- To produce electrical power, for example in power stations or co-generation plants.
- To supply heat for heating and process systems.
When a kilogram of steam condenses completely, a kilogram of condensate is formed at the same pressure and temperature (Figure 14.1.1). An efficient steam system will reuse this condensate.
Failure to reclaim and reuse condensate makes no financial, technical or environmental sense.
Saturated steam used for heating gives up its latent heat (enthalpy of evaporation), which is a large proportion of the total heat it contains. The remainder of the heat in the steam is retained in the condensate as sensible heat (enthalpy of water) (Figure 14.1.2).

As well as having heat content, the condensate is basically distilled water, which is ideal for use as boiler feedwater. An efficient steam system will collect this condensate and either return it to a deaerator, a boiler feedtank, or use it in another process. Only when there is a real risk of contamination should condensate not be returned to the boiler. Even then, it may be possible to collect the condensate and use it as hot process water or pass it through a heat exchanger where its heat content can be recovered before discharging the water mass to drain.
Condensate is discharged from steam plant and equipment through steam traps from a higher to a lower pressure. As a result of this drop in pressure, some of the condensate will re-evaporate into ‘flash steam’. The proportion of steam that will ‘flash off’ in this way is determined by the amount of heat that can be held in the steam and condensate. A flash steam amount of 10% to 15% by mass is typical (see Module 2.2). However, the percentage volumetric change can be considerably more. Condensate at 7 bar g will lose about 13% of its mass when flashing to atmospheric pressure, but the steam produced will require a space some 200 times larger than the condensate from which it was formed. This can have the effect of choking undersized trap discharge lines, and must be taken into account when sizing these lines.
Example 14.1.1 Calculating the amount of flash steam from condensate
Hot condensate at 7 bar g has a heat content of about 721 kJ/kg. When it is released to atmospheric pressure (0 bar g), each kilogram of water can only retain about 419 kJ of heat. The excess energy in each kilogram of the condensate is therefore 721 – 419 = 302 kJ. This excess energy is available to evaporate some of the condensate into steam, the amount evaporated being determined by the proportion of excess heat to the amount of heat required to evaporate water at the lower pressure, which in this example, is the enthalpy of evaporation at atmospheric pressure, 2258 kJ/kg.

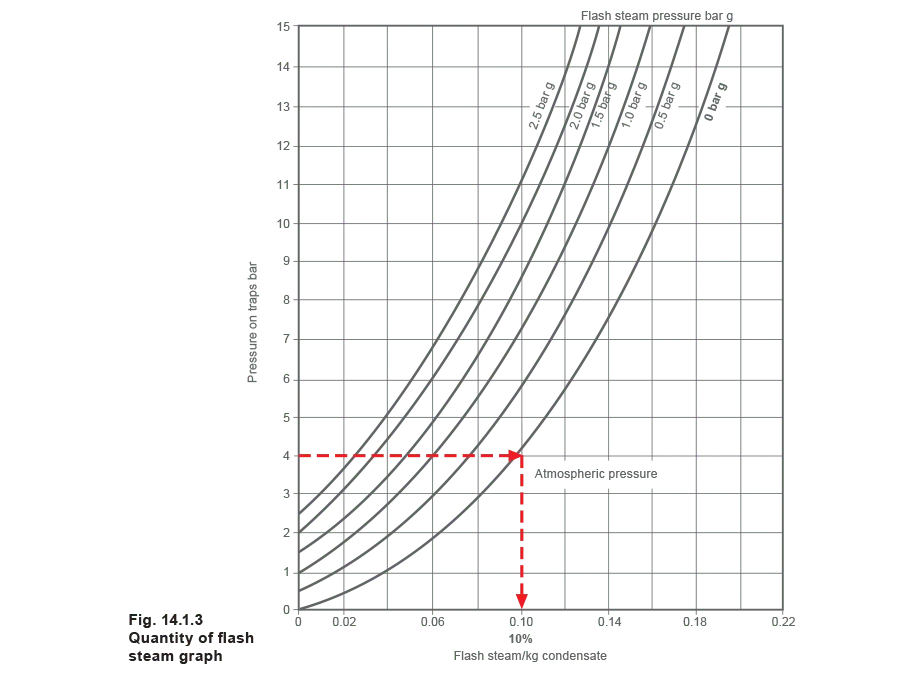
The subject of flash steam is examined in greater depth in Module 2.2, ‘What is steam?’ A simple graph (Figure 14.1.3) is used in this Module to calculate the proportion of flash steam.
Example:
Proportion of flash steam using Figure 14.1.3:
Pressure on the trap = 4 bar g
Flash steam pressure = 0 bar g
% Flash steam = 10%
The amount of flash steam in the pipe is the most important factor when sizing trap discharge lines.
Steam produced in a boiler by the process of adding heat to the water is often referred to as live steam. The terms live steam and flash steam are only used to differentiate their origin. Whether steam is produced in a boiler or from the natural process of flashing, it has exactly the same potential for giving up heat, and each is used successfully for this purpose. The flash steam generated from condensate can contain up to half of the total energy of the condensate. An efficient steam system will recover and use flash steam. Condensate and flash steam discharged to waste means more make-up water, more fuel, and increased running costs.
This Module will look at two essential areas – condensate management and flash steam recovery. Some of the apparent problem areas will be outlined and practical solutions proposed.
Note: The term ‘trap’ is used to denote a steam-trapping device, which could be a steam trap, a pump-trap, or a pump and trap combination. The ability of any trap to pass condensate relies upon the pressure difference across it, whereas a pumping trap or a pump-trap combination will be able to pass condensate irrespective of operational pressure differences (subject to design pressure ratings).
Condensate return
An effective condensate recovery system, collecting the hot condensate from the steam using equipment and returning it to the boiler feed system, can pay for itself in a remarkably short period of time.
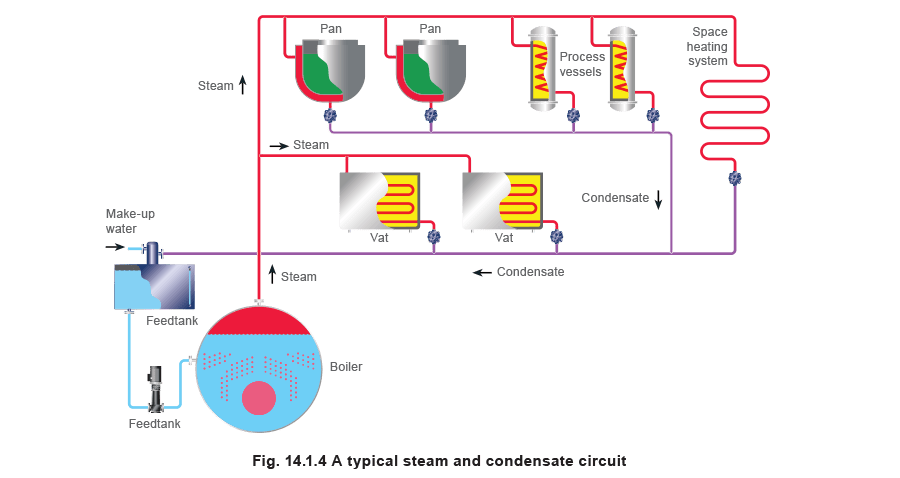
Figure 14.1.4 shows a simple steam and condensate circuit, with condensate returning to the boiler feedtank.
Why return condensate and reuse it?
Financial reasons
Condensate is a valuable resource and even the recovery of small quantities is often economically justifiable. The discharge from a single steam trap is often worth recovering.
Un-recovered condensate must be replaced in the boiler house by cold make-up water with additional costs of water treatment and fuel to heat the water from a lower temperature.
Water charges
Any condensate not returned needs to be replaced by make-up water, incurring further water charges from the local water supplier.
Effluent restrictions
In the UK for example, water above 43°C cannot be returned to the public sewer by law, because it is detrimental to the environment and may damage earthenware pipes. Condensate above thistemperature must be cooled before it is discharged, which may incur extra energy costs. Similar restrictions apply in most countries, and effluent charges and fines may be imposed by water suppliers for non-compliance.
Maximising boiler output
Colder boiler feedwater will reduce the steaming rate of the boiler. The lower the feedwater temperature, the more heat, and thus fuel needed to heat the water, thereby leaving less heat to raise steam.
Boiler feedwater quality
Condensate is distilled water, which contains almost no total dissolved solids (TDS). Boilers need to be blown down to reduce their concentration of dissolved solids in the boiler water. Returning more condensate to the feedtank reduces the need for blowdown and thus reduces the energy lost from the boiler.
Why return condensate and reuse it?
- Summary of reasons for condensate recovery:
- Water charges are reduced.
- Effluent charges and possible cooling costs are reduced.
- Fuel costs are reduced.
- More steam can be produced from the boiler.
- Boiler blowdown is reduced - less energy is lost from the boiler.
- Chemical treatment of raw make-up water is reduced.
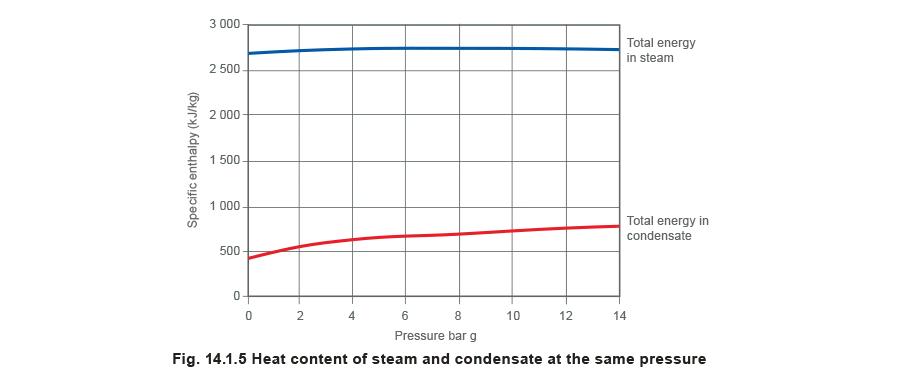
Figure 14.1.5 compares the amount of energy in a kilogram of steam and condensate at the same pressure. The percentage of energy in condensate to that in steam can vary from 18% at 1 bar g to 30% at 14 bar g; clearly the liquid condensate is worth reclaiming.
The following example (Example 14.1.2) demonstrates the financial value of returning condensate.
Example 14.1.2
A boiler produces:
10000 kg/h of steam 24 hours/day, 7 days/week and 50 weeks/year (8400 hours/year).
Raw make-up water is at 10°C. Currently all condensate is discharged to waste at 90°C.
Raw water costs £0.61/m3, and effluent costs are £0.45/m3
The boiler is 85% efficient, and uses gas on an interruptible tariff charged at £0.01/ kWh (£2.77 / GJ).
Steam produced in a boiler by the process of adding heat to the water is often referred to as live steam. The terms live steam and flash steam are only used to differentiate their origin. Whether steam is produced in a boiler or from the natural process of flashing, it has exactly the same potential for giving up heat, and each is used successfully for this purpose. The flash steam generated from condensate can contain up to half of the total energy of the condensate. An efficient steam system will recover and use flash steam. Condensate and flash steam discharged to waste means more make-up water, more fuel, and increased running costs.
This Module will look at two essential areas – condensate management and flash steam recovery. Some of the apparent problem areas will be outlined and practical solutions proposed.
Note: The term ‘trap’ is used to denote a steam-trapping device, which could be a steam trap, a pump-trap, or a pump and trap combination. The ability of any trap to pass condensate relies upon the pressure difference across it, whereas a pumping trap or a pump-trap combination will be able to pass condensate irrespective of operational pressure differences (subject to design pressure ratings).
Condensate return
An effective condensate recovery system, collecting the hot condensate from the steam using equipment and returning it to the boiler feed system, can pay for itself in a remarkably short period of time. Figure 14.1.4 shows a simple steam and condensate circuit, with condensate returning to the boiler feedtank.
Determine the annual value of returning the condensate
Part 1 - Determine the fuel cost
Each kilogram of condensate not returned to the boiler feedtank must be replaced by 1 kg of cold make-up water (10°C) that must be heated to the condensate temperature of 90°C. (ΔT = 80°C).
Calculate the heat required to increase the temperature of 1 kg of cold make-up water by 80°C, by using Equation 2.1.4.
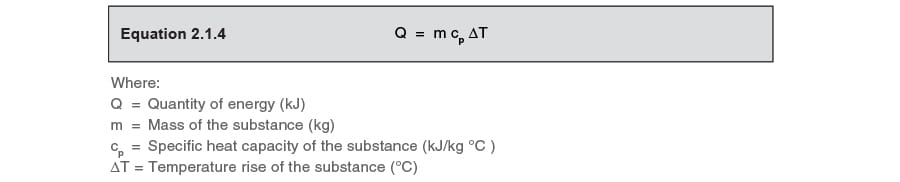
m is unity; ΔT is the difference between the cold water make-up and the temperature of returned condensate; cp is the specific heat of water at 4.19 kJ/kg °C.
1 kg x 4.19 kJ/kg °C x 80°C = 335 kJ/kg
Basing the calculations on an average evaporation rate of 10000 kg/h, for a plant in operation 8 400 h /year, the energy required to replace the heat in the make-up water is:
10 000 kg/h x 335 kJ/kg x 8 400 h/year = 28 140 GJ/year
If the average boiler efficiency is 85%, the energy supplied to heat the make-up water is:

With a fuel cost of £2.77/GJ, the value of the energy in the condensate is:
Annual fuel cost = 33 106 GJ / year x £2.77/GJ = £917 04
Part 2 - Determine the water cost
Water is sold by volume, and the density of water at normal ambient temperature is about 1000 kg /m3. The total amount of water required in one year replacing non-returned condensate is therefore:

If water costs are £0.61 per m³, the annual water cost is:
Annual water cost = 84000 m3/ year x £0.61/m3 = £51 240
Part 3 - Determine the effluent cost
The condensate that was not recovered would have to be discharged to waste, and may also be charged by the water authority.
Total amount of water to waste in one year also equals 84000 m³
If effluent costs are £0.45 per m³, the annual effluent cost is:
Annual effluent cost = 84000 m3/ year x £0.45/m3 = £37 800
Part 4 - Total value of condensate
The total annual value of 10 000 kg/h of condensate lost to waste is shown in Table 14.1.1:
Table 14.1.1 The potential value of returning condensate in Example 14.1.2
| Fuel savings = | £91 704 |
| Water saving = | £51 240 |
| Effluent saving = | £37 800 |
| Total value = | £180 744 |
On this basis, it follows that for each 1% of condensate returned per 10 000 kg/h evaporated as in Example 14.1.2, a saving of 1% of each of the values shown in Table 14.1.1 would be possible.
Example 14.1.3
If it were decided to invest £50 000 in a project to return 80% of the condensate in a similar
plant to Example 14.1.2, but where the total evaporation rate were only 5 000 kg/h, the savings and simple payback term would be:

This sample calculation does not include a value for savings due to correct TDS control and reduced blowdown, which will further reduce water losses and boiler chemical costs. These can vary substantially from location to location, but should always be considered in the final analysis. Clearly, when assessing condensate management for a specific project, such savings must be determined and included.
TDS control and water treatment have already been discussed in Block 3.
The routines outlined in Examples 14.1.2 and 14.1.3 may be developed to form the basis of a forced path calculation to assign a monetary value to projects intended to improve condensate recovery.
Equation 14.1.1 can be used to calculate the fuel savings per year:
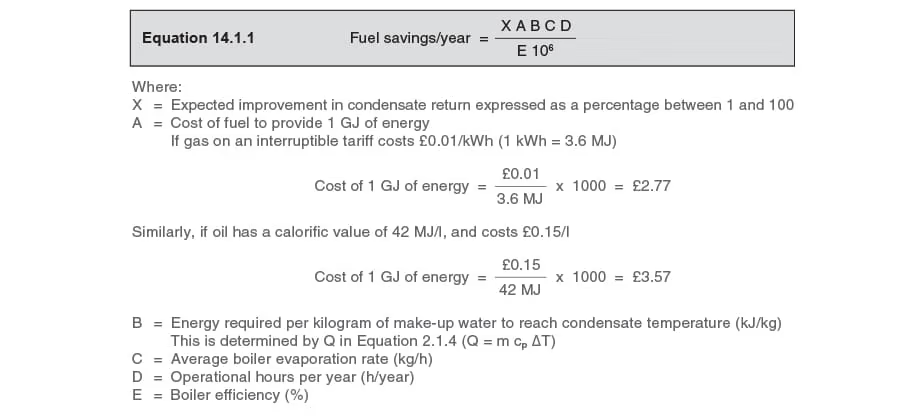
Savings in water costs can be determined using Equation 14.1.2:

Savings in effluent costs can be determined using Equation 14.1.3:

Example 14.1.4
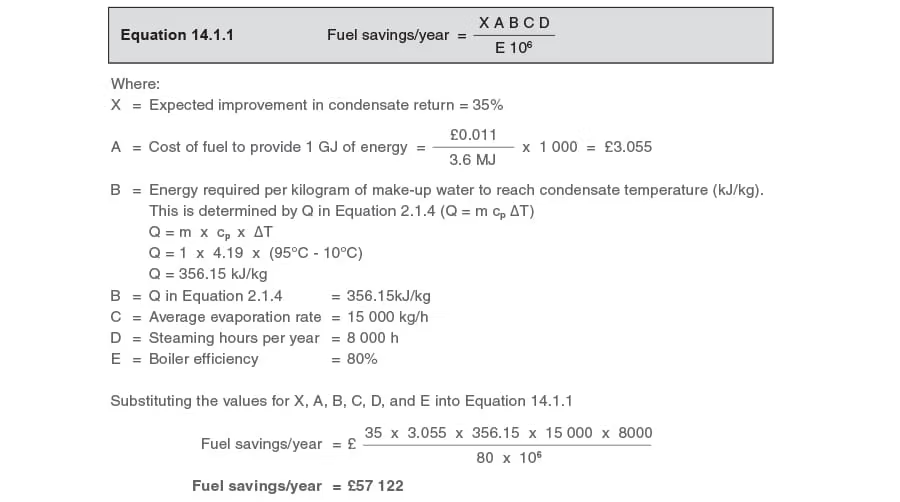
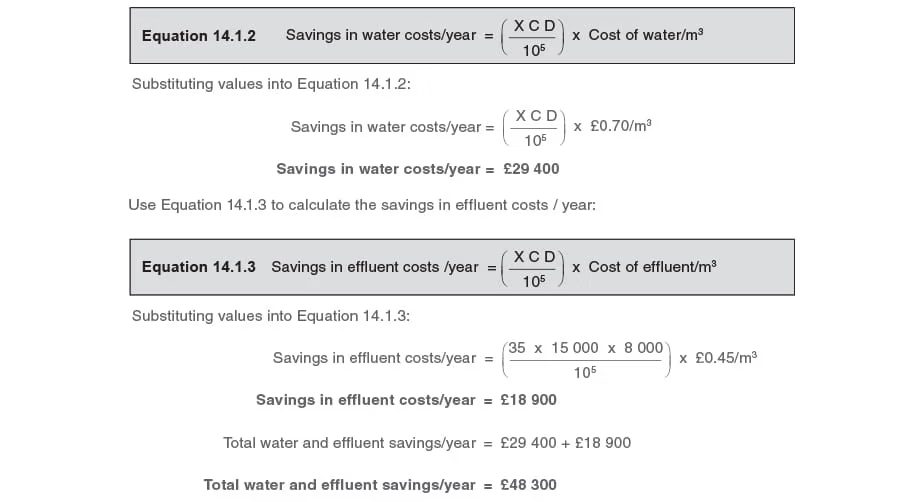
A major condensate management project costing £70 000 expects to recover an additional 35% of the condensate produced at a plant.
The average boiler steaming rate is 15000 kg/h, and the plant operates for 8000 h/year.
The fuel used is gas on a firm tariff of £0.011/ kWh, and the boiler efficiency is estimated as 80%.
Make-up water temperature is 10°C and insulated condensate return lines ensure that condensate will arrive back at the boiler house at 95°C.
Consider the water costs to be £0.70/m3 and the total effluent costs to be £0.45/m3.
Determine the payback period for the project.
Part 1 - Determine the fuel savings
Use Equation 14.1.1:
Part 2 - Determine the water and effluent savings
Use Equation 14.1.2 to calculate the savings in water costs/year:
Part 3 - Determine the payback period

