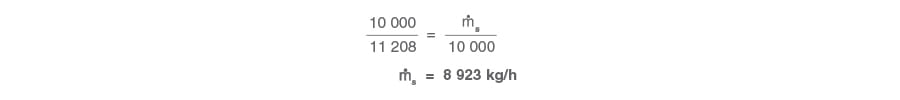Desuperheating

Contents
Basic Desuperheating Theory
Superheated steam has important advantages on certain applications, for example, when used in power stations to drive turbines. For efficient use on heating applications however, the steam must be desuperheated. This tutorial considers basic desuperheating theory and calculations.
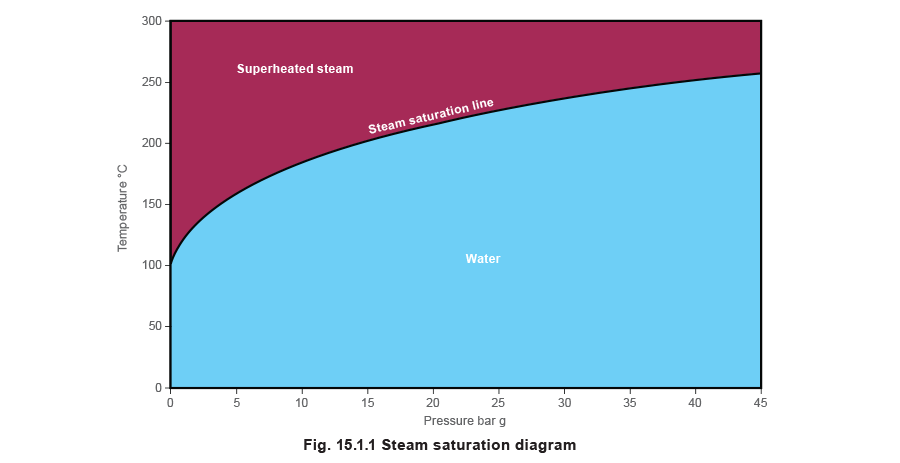
Superheated steam is steam that is at a temperature higher than the saturation temperature for the steam pressure. For example, steam at a pressure of 3 bar g has a saturation temperature of 143.762°C. If further heat were to be added to this steam and the pressure remained at 3 bar g, it would become superheated. This extra heat results in steam which:
- Is higher than saturation temperature.
- Contains more energy than saturated steam.
- Has a greater specific volume than saturated steam.
The relationships between these three properties are well documented and can be found in most texts relating to the thermodynamic properties of steam.
Superheated steam is principally used in power generation plants as the driving force for turbines.
A review of the Rankine gas cycle will demonstrate that, for driving turbines, superheated steam is more thermally efficient than saturated steam.
Superheating the steam has further important advantages:
- Wet steam within a turbine would result in water droplets and erosion of the turbine blades, as well as increased friction.
- Higher pipeline velocities (up to 100 m/s) can be used. This means that smaller distribution pipelines can be used (provided that the pressure drop is not excessive).
- For continuously running plants, superheated steam means there is no condensation in the pipework, therefore, there is only a requirement for steam trapping during start-up.
The use of superheated steam has a number of disadvantages:
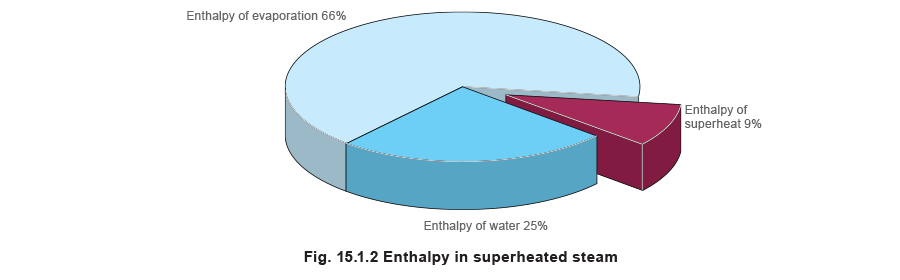
Although superheated steam contains a large amount of heat energy, this energy is in three forms; enthalpy of water, enthalpy of evaporation (latent heat) and enthalpy of superheat. The bulk of the energy is in the enthalpy of evaporation, and the energy in the superheat represents a smaller proportion.
For example, take superheated steam at 10 bar a and 300°C, then:
Enthalpy of water = 763 kJ/kg
Enthalpy of evaporation = 2 015 kJ/kg
Enthalpy of superheat = 274 kJ/kg
- The coefficient of heat transfer when using superheated steam as the heating medium is variable, low and difficult to quantify accurately. This makes accurate sizing and control of heat transfer equipment difficult, and will also result in a larger and more expensive heat exchanger.
Once the superheated steam is cooled to saturation temperature, the heat transfer coefficient increases dramatically, and the temperature at which the steam condenses back into water is constant. This greatly assists accurate sizing and control of heat transfer equipment.
The presence of high heat transfer coefficients associated with saturated steam leads to smaller and cheaper heat exchangers than those which utilise superheated steam.
- Some processes (for example, distillation columns) perform less efficiently when supplied with superheated steam.
- The higher temperatures of superheated steam may mean that higher rated, and hence more expensive equipment is required.
- The higher temperature of superheated steam may damage sensitive equipment.
These disadvantages mean that superheated steam is generally undesirable for thermal process applications. However, sites exist where superheated steam is raised for power generation, and it makes economic sense to desuperheat some of this steam from some point in the power generation cycle, and then use it for process applications. (More information on superheated steam can be found in Module 2.3).
Sites also exist where large quantities of waste are used as fuel for the boiler. If the quantity of waste is sufficiently large, then superheated steam may be produced for power generation.
Examples of this type of plant can be found in the papermaking and sugar refining industries.
In plants that have superheated steam available for process use, it makes sense to distribute the superheated steam to remote points in the plant, as this will ensure that the steam remains dry.
This becomes significant if there are long lengths of pipe separating the point of generation and the point of use.
Basic steam desuperheating
Desuperheating is the process by which superheated steam is restored to its saturated state, or the superheat temperature is reduced.Most desuperheaters used to restore the saturated state produce discharge temperatures approaching saturation (typically to within 3°C of the saturation temperature as a minimum).Designs for discharge temperatures in excess of 3°C above saturation are also possible and often used.
There are basically two broad types of desuperheater:
- Indirect contact type - The medium used to cool the superheated steam does not come into direct contact with it. A cooler liquid or gas may be employed as the cooling medium, for example, the surrounding air. Examples of this type of desuperheater are shell and tube heat exchangers.
Here the superheated steam is supplied to one side of the heat exchanger and a cooler medium is supplied to the other side. As the superheated steam passes through the heat exchanger, heat is lost from the steam, and gained by the cooling medium.
The temperature of the desuperheated steam could be controlled by either the inlet superheated steam pressure or the flowrate of the cooling water. Control of the superheated steam flow for this purpose is not normally practical and most systems adjust the flow of the cooling medium.
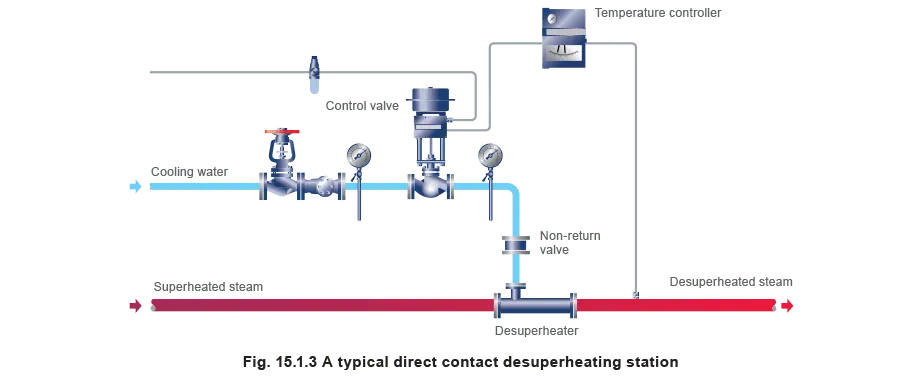
- Direct contact type - The medium used to cool the superheated steam comes into direct contact with it. In most cases, the cooling medium is the same fluid as the vapour to be desuperheated, but in the liquid state. For example, in the case of steam desuperheaters, water is used. A typical direct contact desuperheating station is shown in Figure 15.1.3.
When the desuperheater is operational, a measured amount of water is added to the superheated steam via a mixing arrangement within the desuperheater. As it enters the desuperheater, the cooling water evaporates by absorbing heat from the superheated steam. Consequently, the temperature of the steam is reduced.
Control of the amount of water to be added is usually achieved by measuring the temperature of the steam downstream of the desuperheater. The set temperature of the desuperheated steam would typically be 3°C above that at saturation. Therefore, in such arrangements the inlet pressure of the superheated steam should be kept constant.
Desuperheating calculations
The amount of water added must be sufficient to cool the steam to the desired temperature; too little water and the steam will not have been cooled enough, too much and wet saturated steam will be produced which will require drying through a separator.
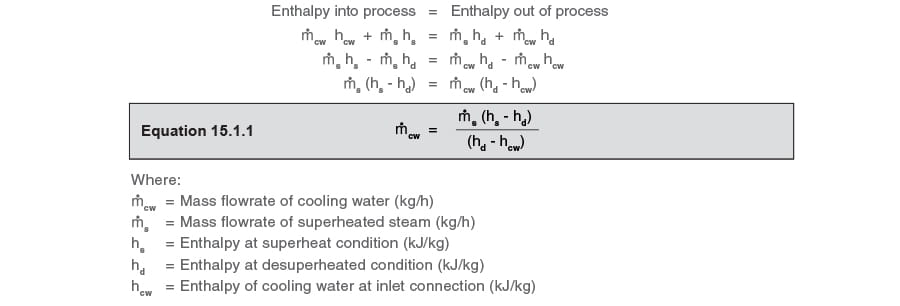
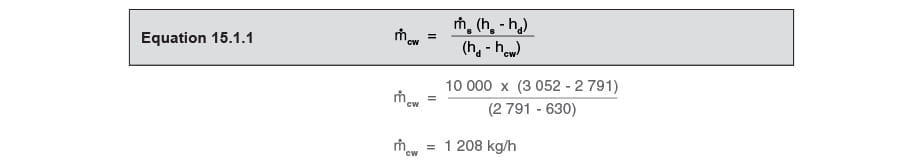
Using Equation 15.1.1, which is based on the conservation of energy, the cooling liquid requirement can be easily and quickly determined:
Example 15.1.1
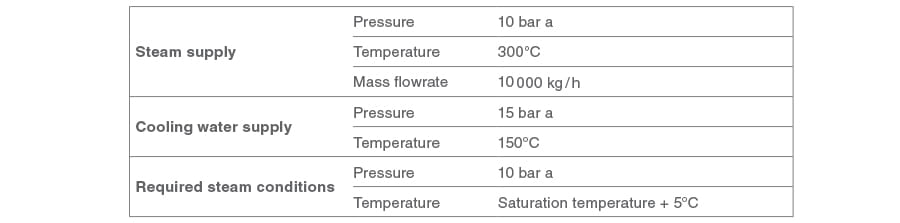
Determine the required cooling water flowrate for the conditions in the following Table:
Solution:
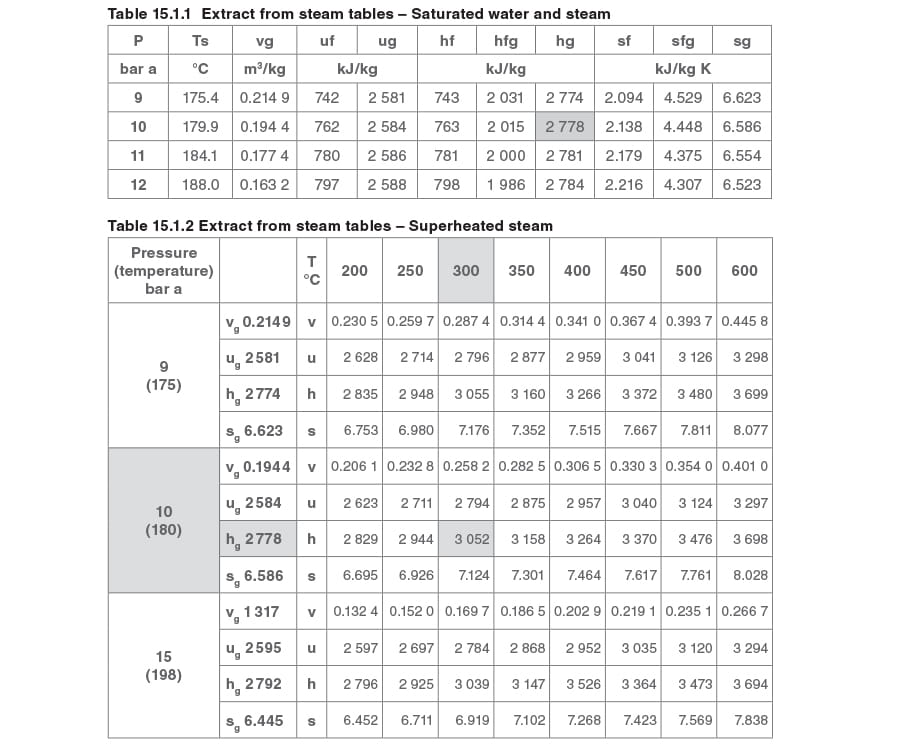
The necessary information can be obtained or interpolated from hard copy steam tables; the relevant extracts are shown in Table 15.1.1 and Table 15.1.2. Alternatively, the Spirax Sarco online steam tables can be used.
The information required to satisfy Equation 15.1.1 is therefore:
m_dot - body text.jpgs = Mass flowrate of superheated steam = 10 000 kg/h
hs = Enthalpy at superheat condition (From steam tables 300°C at 10 bar a) = 3 052 kJ/kg
hcw = Enthalpy of the cooling liquid = 4.2 kJ/kg°C x 150°C =630 kJ/kg
Determining the enthalpy at the desuperheated condition, hd:
From steam tables, the saturation temperature (Ts) at 10 bar a is 180°C, therefore at the required desuperheated condition, the temperature will be:
Ts + 5°C = 185°C
Interpolating between the enthalpy of steam at 10 bar a and its saturation temperature, and at 10 bar a and 200°C:
Enthalpy at 10 bar a, Ts (saturated steam tables) = 2 778 kJ/kg
Enthalpy at 10 bar a, 200°C (superheated steam tables) = 2 829 kJ/kg
Interpolating for enthalpy at 10 bar a and 185°C:
Finally, applying Equation 15.1.1:
Note that the desuperheated steam is supplied at a rate of: 10 000 + 1 208 kg/h = 11 208 kg/h
supplied at a rate of:
10 000 + 1 208 Had the requirement been for 10 000 kg/h of the desuperheated steam, the initial superheated steam flowrate can be determined using a simple proportional method: